Ozone (O3), called "activated oxygen," contains three atoms of oxygen rather than the two atoms we normally breathe. Ozone is the second most powerful sterilant in the world and can be used to destroy bacteria, viruses and odors. Interestingly, ozone occurs quite readily in nature, most often as a result of lightning strikes that occur during thunderstorms.
Ozone operates according to the principle of oxidation. The third oxygen atom of ozone is extremely reactive because it is unstable. When the static loaded ozone molecule (O3) contacts with something oxidizable, the charge of the ozone molecule will directly flow over. Ozone can oxidize with all kinds of materials, but also odor and microorganisms like viruses, moulds and bacteria. The extra oxygen atom releases from the ozone molecule and binds with the other material. Eventually remains only the pure and stable oxygen molecule.
Ozone can be produced artificially according to the same principle as it occurs in nature.
- Corona Discharge - It creates ozone by applying high voltage to a metallic grid sandwiched between two dielectrics. The high voltage jumps through the dielectric to a grounded screen and in the process, creates ozone from oxygen present in the chamber. This also occurs naturally during lightning storms.
- Ultra-violet UV - UV light creates ozone when a wavelength at 254 nm (nanometers) hits an oxygen atom. The molecule (O2) splits into two atoms (O) which combine with another oxygen molecule (O2) to form ozone (O3). This also occurs naturally through the sun rays.
Ozone will last between 30 minutes to 4 hours for the third oxygen atom to break apart to react with odors and bacteria, and revert back into oxygen. On a lower concentration level, it will typically take 30 minutes to 1-2 hours for ozone to break down into breathable oxygen. Additionally ozone breaks down thermally. Higher temperatures destroy ozone quicker than lower temperatures.
Ozone is destroyed or self-destructed, it can return back to oxygen. Destruction of ozone requires energy since it is an exothermic reaction. Destruction of ozone is carried out by different methods.
- Adsorption process
- Catalytic process
- Chemical dilution
- Thermal decomposition
Ozone is considered as fully utilized or destroyed when the off gas is 0.12 ppm in the outdoor and 0.1 ppm in the indoor environment.
Ozone is used to oxidize anything that is oxidizable. Oxidation is a chemical reaction in which electrons are lost by ions,atoms or molecules.
Ozone is harmful after inhalation when it is used at higher concentrations. 0.1 PPM is the maximum concentration allowable in the presence of humans for 8 hours in a day, similarly 5 days in a week. This limit was proposed by occupational safety and health agency (OSHA). The concentrations mentioned above are much higher than the odour threshold at which ozone can be smelled, so critical concentrations will be noticed quickly. When people are exposed to high ozone concentrations the symptoms can vary from dryness in the mouth and throat, coughing, headache and chest restriction. Nearby the lethal limits, more acute problems will follow. When larger ozone generators are applied, ozone destructors can be used for the destruction of residual ozone.
- Ozone leaves no chemical by-products in water
- Ozone leaves no chemical taste or smell
- Ozone will not burn eyes or make them red or irritated
- Ozone will not irritate or dry out skin, nose or ears
- Ozone will not discolour or damage hair or clothing
- Ozone adds no contaminants or by-products to water
- Ozone rids water and air of unhealthy micro-organisms
- Ozone is NOT a carcinogen
There are a lot of measuring instruments available to measure ozone in water and air. These measuring instruments rest on different principles and can measure the concentrations from PPM (PPM = Parts Per Million) to PPB (= Parts Per Billion). The instruments can be used for monitoring and controlling the ozone generator.
- Ozone destroys bacteria, viruses, mold and mildew
- Ozone eliminates spores, cysts, yeast and fungus
- Ozone oxidizes iron, sulfur, manganese and hydrogen sulfate
- Ozone eliminates oils and other contaminants in water
- Ozone keeps water clean and sparkling clear
- Ozone keeps water fresh
Ozone kills bacteria by destroying the cell wall of the bacteria. Once the cell wall is destroyed, the bacteria will be unable to survive.
Ozone destroys viruses by diffusing through the protein coat into the nucleic acid core, resulting in damage of the viral RNA. At higher concentrations, ozone destroys the exterior protein shell by oxidation.
Ozone destroys microorganisms instantly and effectively without leaving harmful residue in treated air, food or processing water, unlike other chemicals used for the same purpose. Therefore, ozone is safer and environmentally friendlier than most other antimicrobials. For example, ozone kills bacteria 3,125 times faster than chlorine.


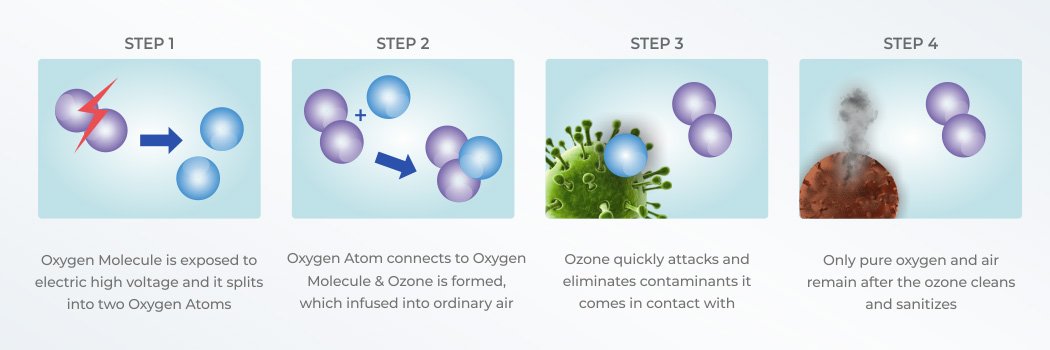
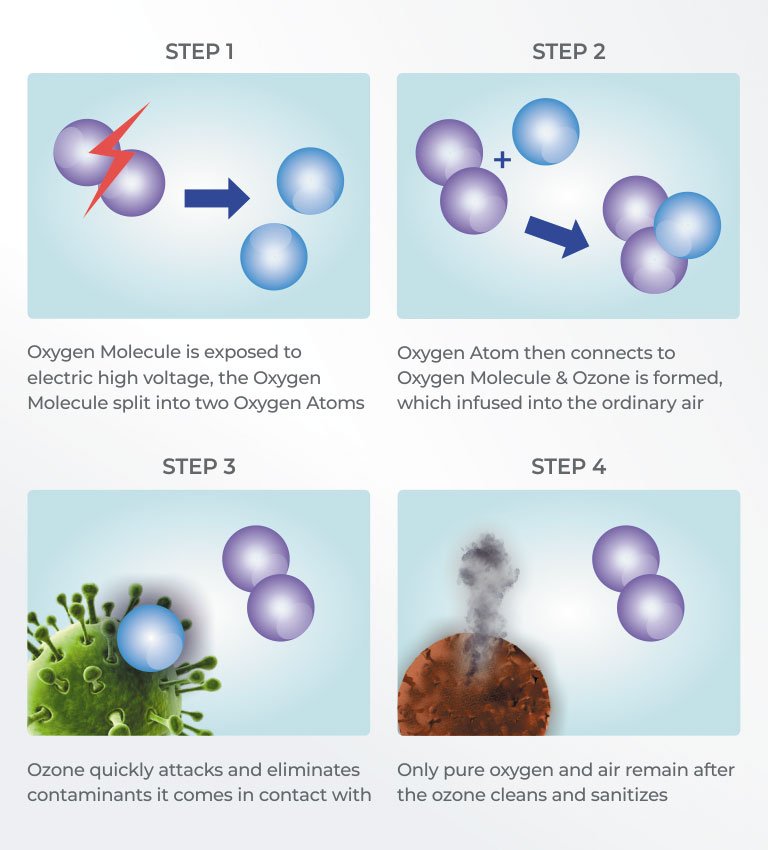 The ozone is injected into the water or air stream, where it inactivates contaminants by actually
rupturing the organism call wall. At the heart of a corona discharge ozone system is the
dielectric. The electrical charge is diffused over this dielectric surface, creating
an electrical surface, creating an electrical field or "Corona".
The ozone is injected into the water or air stream, where it inactivates contaminants by actually
rupturing the organism call wall. At the heart of a corona discharge ozone system is the
dielectric. The electrical charge is diffused over this dielectric surface, creating
an electrical surface, creating an electrical field or "Corona".